Show Menu
Choose Your Location
You are now leaving the Viatris page for a Viatris affiliate site or third party site that is solely responsible for its content, including its compliance with guidelines applicable in certain geographies. Links to Viatris affiliate sites and third party sites are provided as a resource to our visitors and may not be governed by the same regulatory requirements applicable to this site and unaffiliated third party sites are subject to their own terms and data protection notices and practices. Moreover, if their third party site is subject to other country laws, regulatory requirements, data protection requirements or medical practices may differ between countries and the information provided therein may not be suitable for use in your country.

On World TB Day, we reflect on the progress made and need to do more in the fight against drug-resistant TB.
March 24, 2021
Each year, March 24 is dedicated to acknowledging the incredible progress that has been made in the fight against tuberculosis (TB) while also recognizing that we all must remain steadfast and committed to do more. The past decade has seen a nearly 20% reduction in the number of global deaths from TB[1], clear evidence that global efforts are advancing care and treatment for patients. However, despite these improvements, TB remains one of the world’s deadliest infectious diseases, claiming 1.4 million lives – the vast majority in low- and middle-income countries (LMICs).
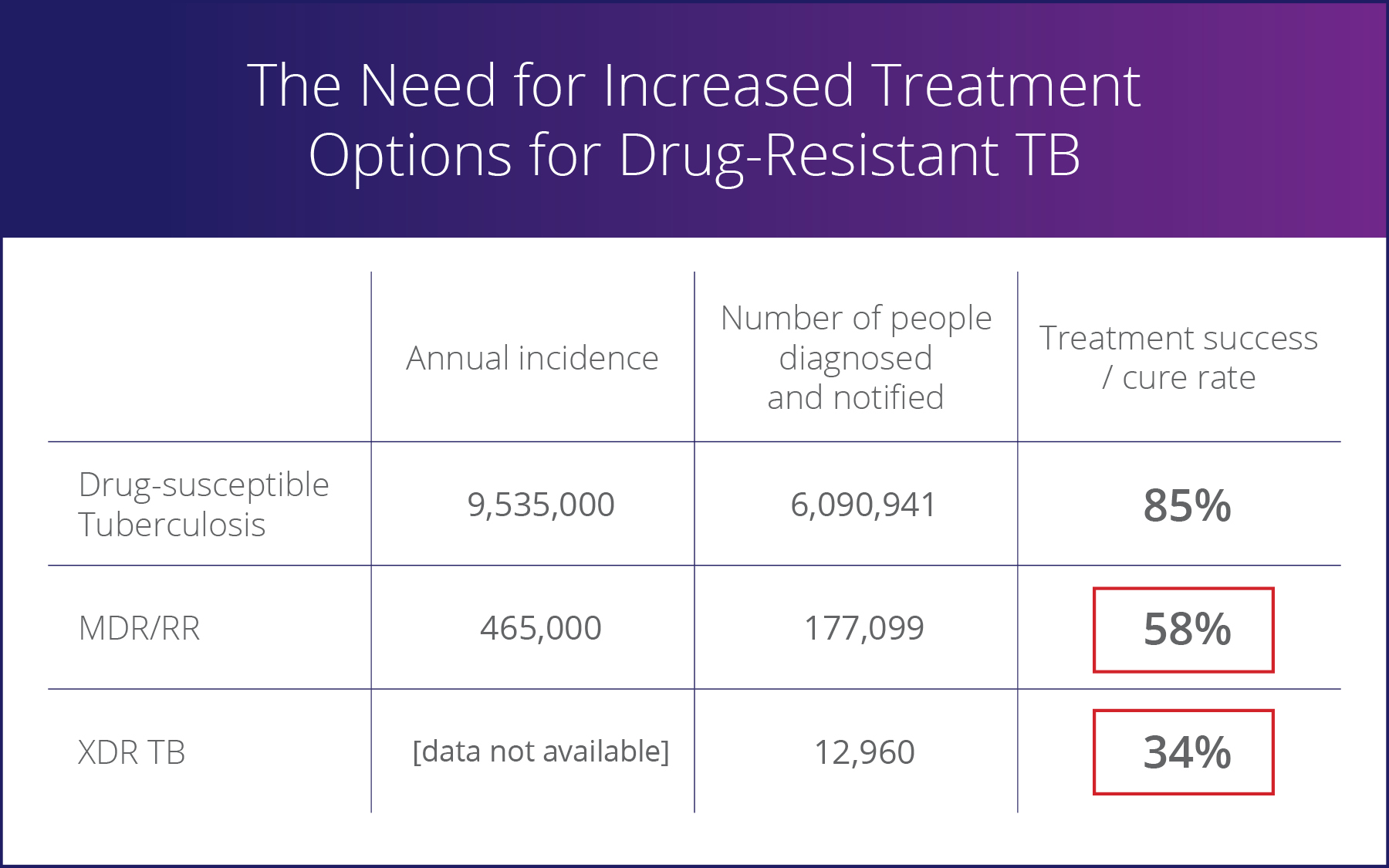
Multi-drug resistant/rifampicin-resistant (MDR/RR) and extensively drug-resistant (XDR) TB, together commonly referred to as DR TB, represent the greatest threat to the progress that has been made. While MDR/RR and XDR-TB cases represent less than 5% of the total global TB cases, they account for nearly 15% of all TB deaths. DR TB also is a major factor in the growth of anti-microbial resistance (AMR). It’s estimated that DR TB cases will account for more than one-quarter of all deaths from AMR over the next 25 years[2].
Addressing an Unmet Need
Despite the significant global burden of TB, research into new therapies has historically been neglected. Patients with DR TB have had limited options and are often treated with a complicated mix of drugs with a single course of treatment taking 18 to 24 months, five to seven different medicines and thousands of tablets. These treatments include debilitating side effects, including the potential risk of hearing loss.
At Viatris, we saw the status quo as unacceptable and took this as a challenge to identify an innovative solution. Moreover, we realized that we had a powerful platform, as the world’s largest producer of HIV drugs by volume, to help those living with TB around the world, including the regions where the TB burden is the highest – in low- and lower-middle income countries.
That’s why, in 2019 through one of our predecessor companies, we joined the nonprofit TB Alliance as its global commercialization partner for the first drug approved to treat XDR-TB or MDR/RR-TB that is treatment-intolerant or non-responsive.
“All people suffering from TB must have access to innovation. That is why we are proud to partner with Viatris and leverage their deep experience in delivering high quality, affordable medicines all over the world,” said Dr. Mel Spigelman, president and CEO, TB Alliance. “No treatment can make a difference if it doesn’t reach people in need. We are encouraged by the rapid pace of access to this regimen and look forward to achieving public health impact on a global scale.”
This important treatment was developed by TB Alliance from early discovery through its first regulatory approval. It has since been approved over the past two years by several global regulatory health authorities, including the Drug Controller General of India (DCGI), where the prevalence of TB is the highest in the world.
Increasing Global Access to Treatment
At Viatris, a core focus of our mission is to help provide access to medicines, regardless of geography or circumstance. Within two months of the first regulatory approval, we were able to make treatment available to public health programs in 150 LMICs through the StopTB Partnership’s Global Drug Facility for $2 a day. For the first time in history, a full treatment regimen, including a combination of three products, for highly drug-resistant TB is available for under $1,000 per full treatment course.
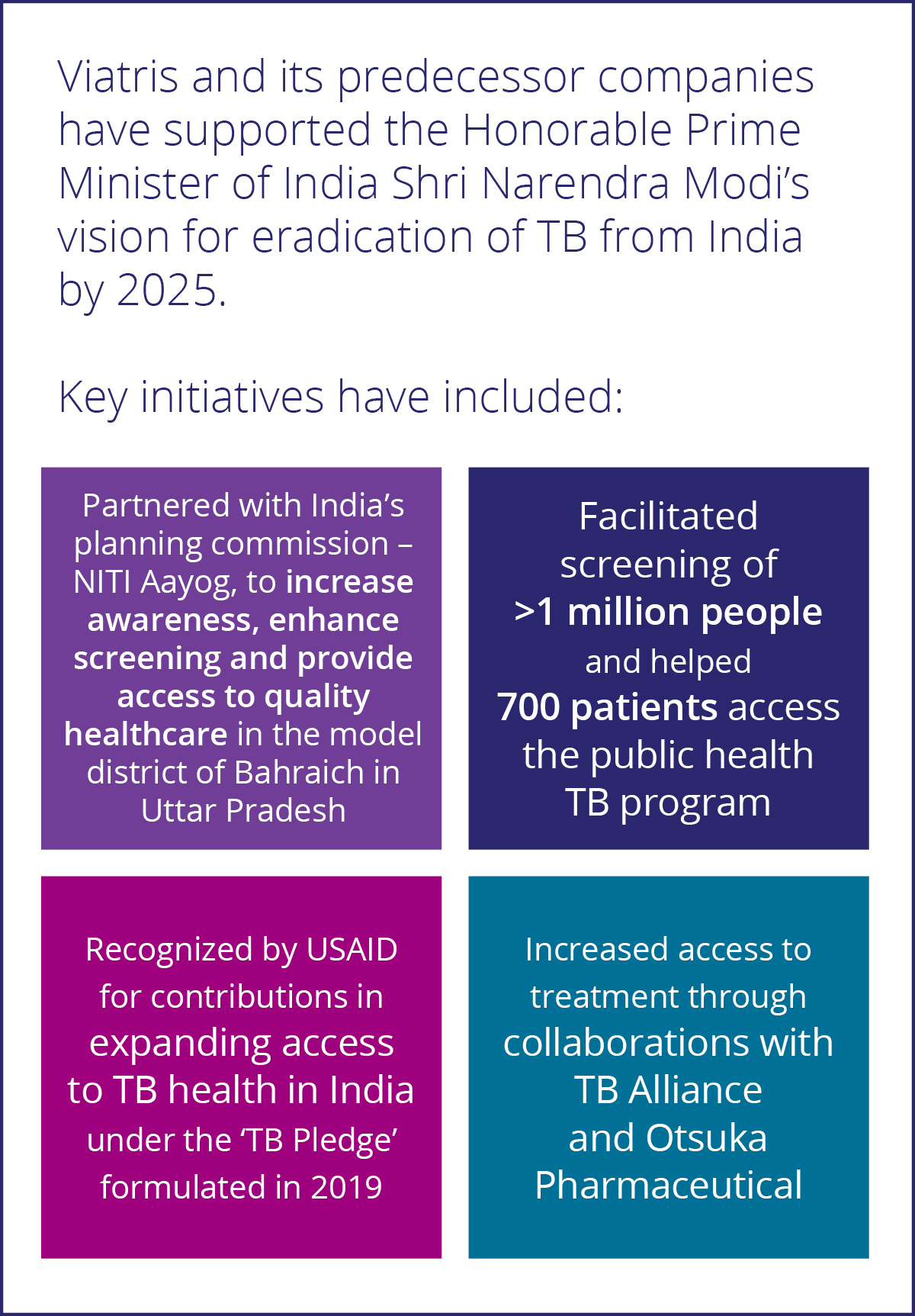
In addition, to reach patients living in countries where the drug was not registered or available, Viatris developed a Named Patient Access Program to ensure that any physician, regardless of where they live, can access this product free of charge as long as their patient meets certain eligibility criteria.
The registration of the product in additional LMICs also continues to advance rapidly, with the filing of the product in close to 30 countries to ensure that treatment is accessible at the same time it is available in high-income countries. In India, for example, regulatory approval was received in less than 12 months after the first regulatory approval in a developed market. This speed-to-market marked a new record, as other recently developed MDR-TB drugs have taken more than three years to secure product registration.
“Given our deep history and commitment in HIV and understanding of TB as the leading cause of death among people living with HIV, we recognized the opportunity and urgent need to apply our learnings and experience to assist those impacted by this serious disease. We’re proud to partner with TB Alliance and others around the world to make critical treatments available. We are setting new standards for accelerating the pace of access and delivering treatments to patients in the greatest need,” said Viatris President Rajiv Malik.
“This work also is a strong example of how we’re executing and optimizing our Global Healthcare Gateway® as a Partner of Choice™ to connect products and people and expand access to medicines worldwide,” Malik added.
Finally, in partnership with TB Alliance, we’re also advancing key operational research and conditional access programs around the world. We are now actively working with not-for-profit organizations and governments across more than a dozen countries in Eastern Europe, Central Asia, South and Southeast Asia and sub-Saharan Africa to further evaluate the safety and efficacy of the treatment. We believe that these efforts are crucial, not only in advancing the World Health Organization’s recommendations but to ensure that countries, physicians and patients gain real-world experience.
These steps are just the beginning in the global fight and how we are redefining the healthcare landscape for those affected by TB. We know we can do more – and are committed to do more – to increase access to treatment and make a difference for those impacted by TB.
[1] 2009: https://apps.who.int/iris/bitstream/handle/10665/44425/9789241564069_eng.pdf?sequence=1